A nitrile is an organic
chemical that contains a cyano functional group (subunit), CN-,
in which the carbon and nitrogen atoms have a triple bond i.e. C≡N-.
The general chemical formula of a nitrile is RCN, where R is the organic group.
Additional
Information
Under the standard
chemical naming convention used by chemists (IUPAC nomenclature), nitriles
are named according to these rules:
a. Compounds
in which the carbon atom of the CN group is attached to an acyclic
hydrocarbon fragment are generally named using the suffix nitrile.
b. Nitriles
in which the -CN group can be thought of as having replaced the -COOH group
of a corresponding trivially named carboxylic acid are named by removing
"ic acid" or "oic acid" and replacing it with onitrile.
c. A
nitrile that is an analog of a compound that has "carboxylic acid"
as its suffix, is named by subsitituting the suffix carbonitrile
|
In addition, the use of cyanide
as a suffix is still common, and one nitrile may be known by a variety of
different names. Some examples of these are shown below, with the most rigorous
IUPAC name for each shown in red:
When there are other
functional groups that take naming precedence over the cyanide/nitrile group,
the prefix cyano is used. In this example, we have color-coded the parts
of the name and the parts of the molecule that correspond to each other:
Physical
properties
The small nitriles are
liquids at room temperature.
nitrile
|
boiling point (°C)
|
CH3CN
|
82
|
CH3CH2CN
|
97
|
CH3CH2CH2CN
|
116 - 118
|
These boiling points are
very high for the size of the molecules - similar to what you would expect if
they were capable of forming hydrogen bonds.
However, they don't
form hydrogen bonds - they don't have a hydrogen atom directly attached to an
electronegative element.
They are just very polar
molecules. The nitrogen is very electronegative and the electrons in the triple
bond are very easily pulled towards the nitrogen end of the bond.
Nitriles therefore have
strong permanent dipole-dipole attractions as well as van der Waals dispersion
forces between their molecules.
Solubility
in water
Ethanenitrile is completely
soluble in water, and the solubility then falls as chain length increases.
nitrile
|
solubility at 20°C
|
CH3CN
|
miscible
|
CH3CH2CN
|
10 g per 100 cm3
of water
|
CH3CH2CH2CN
|
3 g per 100 cm3
of water
|
The reason for the
solubility is that although nitriles can't hydrogen bond with themselves, they can
hydrogen bond with water molecules.
One of the slightly positive
hydrogen atoms in a water molecule is attracted to the lone pair on the
nitrogen atom in a nitrile and a hydrogen bond is formed.
There will also, of course,
be dispersion forces and dipole-dipole attractions between the nitrile and
water molecules.
Forming these attractions releases
energy. This helps to supply the energy needed to separate water molecule from
water molecule and nitrile molecule from nitrile molecule before they can mix
together.
As chain lengths increase,
the hydrocarbon parts of the nitrile molecules start to get in the way.
By forcing themselves
between water molecules, they break the relatively strong hydrogen bonds
between water molecules without replacing them by anything as good. This makes
the process energetically less profitable, and so solubility decreases.
MAKING
NITRILES
Making
nitriles from halogenoalkanes
The halogenoalkane is heated
under reflux with a solution of sodium or potassium cyanide in ethanol. The
halogen is replaced by a -CN group and a nitrile is produced. Heating under
reflux means heating with a condenser placed vertically in the flask to prevent
loss of volatile substances from the mixture.
The solvent is important. If
water is present you tend to get substitution by -OH instead of -CN.
For example, using
1-bromopropane as a typical halogenoalkane:
You could write the full
equation rather than the ionic one, but it slightly obscures what's going on:
The bromine (or other
halogen) in the halogenoalkane is simply replaced by a -CN group - hence a
substitution reaction. In this example, butanenitrile is formed.
Making a nitrile by this
method is a useful way of increasing the length of a carbon chain. Having made
the nitrile, the -CN group can easily be modified to make other things - as you
will find if you explore the nitriles menu (link a the bottom of the page).
Making
nitriles from amides
Nitriles can be made by
dehydrating amides.
Amides are dehydrated by
heating a solid mixture of the amide and phosphorus(V) oxide, P4O10.
Water is removed from the
amide group to leave a nitrile group, -CN. The liquid nitrile is collected by
simple distillation.
For example, you will get
ethanenitrile by dehydrating ethanamide.
Making
nitriles from aldehydes and ketones
Aldehydes and ketones
undergo an addition reaction with hydrogen cyanide. The hydrogen cyanide adds
across the carbon-oxygen double bond in the aldehyde or ketone to produce a
hydroxynitrile. Hydroxynitriles used to be known as cyanohydrins.
For example, with ethanal
(an aldehyde) you get 2-hydroxypropanenitrile:
With propanone (a ketone)
you get 2-hydroxy-2-methylpropanenitrile:
In every example of this
kind, the -OH group will be on the number 2 carbon atom - the one next to the
-CN group.
The reaction isn't normally
done using hydrogen cyanide itself, because this is an extremely poisonous gas.
Instead, the aldehyde or ketone is mixed with a solution of sodium or potassium
cyanide in water to which a little sulphuric acid has been added. The pH of the
solution is adjusted to about 4 - 5, because this gives the fastest reaction.
The reaction happens at room temperature.
The solution will contain
hydrogen cyanide (from the reaction between the sodium or potassium cyanide and
the sulphuric acid), but still contains some free cyanide ions. This is
important for the mechanism.
These are useful reactions
because they not only increase the number of carbon atoms in a chain, but also
introduce another reactive group as well as the -CN group. The -OH group
behaves just like the -OH group in any alcohol with a similar structure.
For example, starting from a
hydroxynitrile made from an aldehyde, you can quite easily produce relatively
complicated molecules like 2-amino acids - the amino acids which are used to
construct proteins.
The
use of Nitriles
Nitriles have tremendous
industrial importance. For example, through a process called hydrocyanation,
HCN reacts with 1,4-butadiene (an alkene) to form adiponitrile
(1,4-dicyanobutane, hexanedinitrile, tetramethylene cyanide, NC(CH2)4CN),
a chemical precursor to hexamethylene diamine (1,6-diaminohexane, H2N(CH2)6NH2),
one of the polymers used to make nylon. The wide range of chemical reactivity
for nitriles is what makes them so useful, but this same feature means that
they are incompatible with many substances (see MSDS relevance below).
~~forgiveness if there are
mistakes~~
Problem
Cyanide is
highly toxic. Cyanide consists
of cyano, CN-,
so does the nitrile.
whether nitrile is also highly toxic ??
whether nitrile is also highly toxic ??



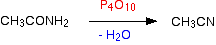


You can say that again....
BalasHapusBut I will try to solve your problem. according the refference that i read i got "Any chemical compound containing the combining group CN. Ionic ( ion; ionic bond) and organic cyanide compounds differ in chemical properties, but both are toxic, especially the ionic ones. Cyanide poisoning inhibits cells' oxidative ( oxidation-reduction) processes; its action is extremely rapid, and an antidote must be given promptly. Cyanides occur naturally in certain seeds (e.g., apple seeds, wild cherry pits). Cyanides, including hydrogen cyanide (HCN, or hydrocyanic acid), are used industrially in the production of acrylic fibres, synthetic rubbers, and plastics as well as in electroplating, case-hardening of iron and steel, fumigation, and concentration of ores.
Read more: http://www.answers.com/topic/cyanide#ixzz1xV2gY8pI" I hope you will get benefit
nitrile compound is a carboxylic acid derivative having the structure R-CN. and in general are highly toxic due to the nitrile group sianidanya and some are mutagenic, carcinogenic, and feratogenik. but Nitriles are usually less toxic than cyanide salts.
BalasHapusNitrile group-containing compounds are highly toxic because it can remove the CN ion.
BalasHapusSynthesis of nitrile compounds was first carried out by KW Scheele in 1782. The compound obtained is asamformiat nitrile, hydrogen cyanide. Gay-Lussac in 1811, JL was able to make a nitrile acid is highly toxic and volatile with a high degree of purity. Synthesis benzonitril by Hermann Fehling in 1844 by heating ammonium benzoate is the first method that produces a lot of research on chemical substances. He determined the structure by comparing the results of the synthesis of hydrogen cyanide that has been identified by heating ammonium formic into the results of his experiments